Docetaxel refined for today's treatment standards
Combines docetaxel + human-derived albumin, the body’s most abundant natural carrier protein3,4
Free of polysorbate-80 and other synthetic solvents which could have an impact on treatment consistency2

(not actual patient)
The body’s most abundant plasma protein 4
Highly suitable for drug delivery 4
Beizray: Docetaxel + Albumin3

Beizray (PS-80 free docetaxel) and albumin are combined in 0.9% saline IV solution*

Beizray molecules disperse in the blood stream*
Docetaxel is an antineoplastic agent3 that acts by disrupting the microtubular network in cells that is essential for mitotic and interphase cellular functions.
Beizray is supplied as a
docetaxel + albumin kit*
160 mg Kit:
Two single-dose vials of docetaxel injection: 80 mg/4 mL each
One single-dose vial of IV Solution stabilizer: 50 mL of 25% Human Albumin solution for infusion
80 mg Kit:
One single-dose vial of docetaxel injection: 80 mg/4 mL
One single-dose vial of IV Solution stabilizer: 50 mL of 25% Human Albumin solution for infusion
*Also supplied individually as an 80 mg/4 mL single dose vial
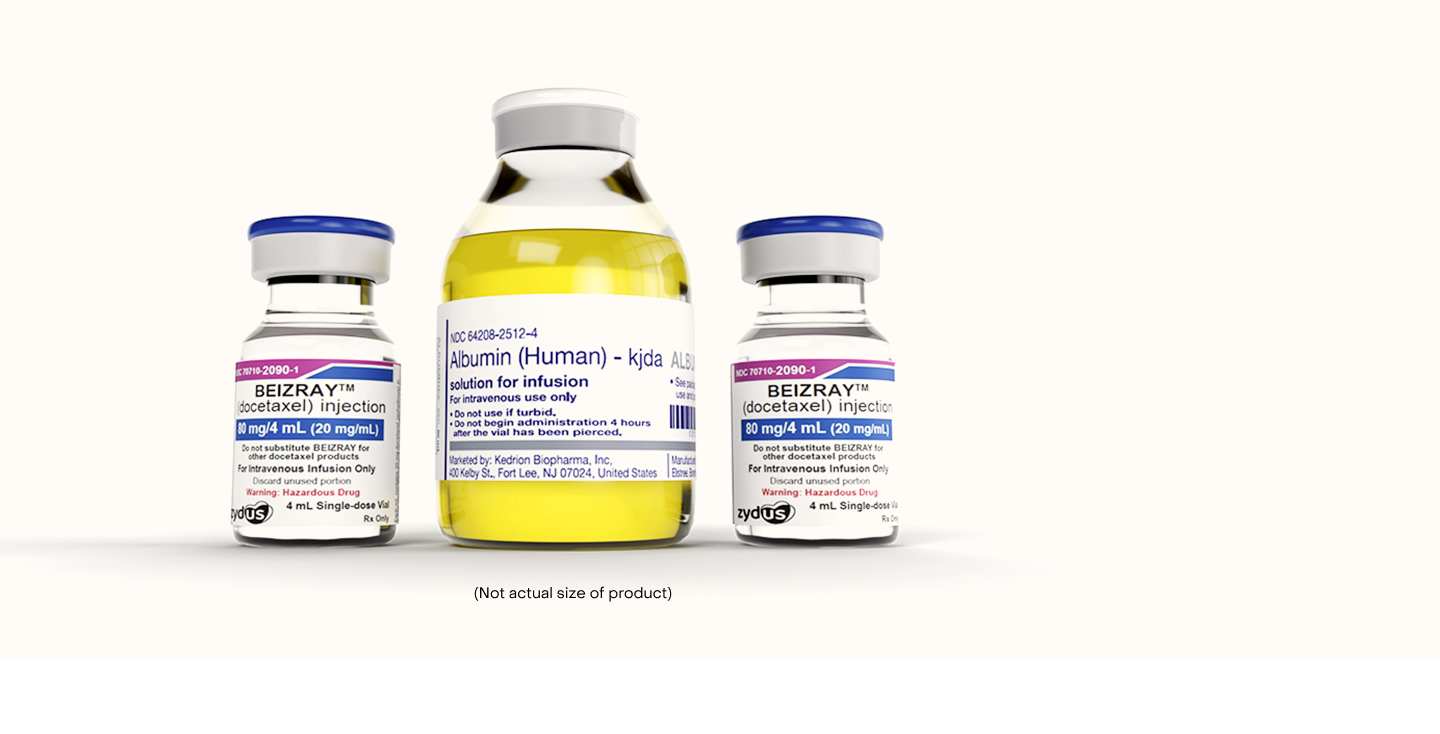
(Not actual size of product)
160 mg Kit:
Two single-dose vials of docetaxel injection: 80 mg/4 mL each
One single-dose vial of IV Solution stabilizer: 50 mL of 25% Human Albumin solution for infusion
80 mg Kit:
One single-dose vial of docetaxel injection: 80 mg/4 mL
One single-dose vial of IV Solution stabilizer: 50 mL of 25% Human Albumin solution for infusion
*Also supplied individually as an 80 mg/4 mL single dose vial
Beizray has demonstrated bioequivalence to Taxotere1
For locally advanced or metastatic breast cancer after failure of prior chemotherapy, the recommended dosage of BEIZRAY is 60 mg/m² to 100 mg/m² administered intravenously over 1 hour every 3 weeks.
For the adjuvant treatment of operable node-positive breast cancer, the recommended BEIZRAY dosage is 75 mg/m² administered 1 hour after doxorubicin 50 mg/m² and cyclophosphamide 500 mg/m² every 3 weeks for 6 cycles.
For treatment after failure of prior platinum-based chemotherapy, the recommended dosage of BEIZRAY monotherapy is 75 mg/m² administered intravenously over 1 hour every 3 weeks.
In patients previously treated with chemotherapy, a dosage of 100 mg/m² is not recommended because this dosage was associated with increased hematologic toxicity, infection, and treatment-related mortality in randomized controlled trials.
For chemotherapy-naive patients, the recommended dosage of BEIZRAY is 75 mg/m² administered intravenously over 1 hour immediately followed by cisplatin 75 mg/m² over 30–60 minutes every 3 weeks.
For metastatic castration-resistant prostate cancer, the recommended dosage of BEIZRAY is 75 mg/m² every 3 weeks as a 1-hour intravenous infusion.
Recommend concomitant use of 5 mg of prednisone orally twice daily continuously.
For gastric adenocarcinoma, the recommended dosage of BEIZRAY is 75 mg/m² as a 1-hour intravenous infusion, followed by cisplatin 75 mg/m² as a 1 to 3 hour intravenous infusion (both on day 1 only), followed by fluorouracil 750 mg/m² per day given as a 24-hour continuous intravenous infusion for 5 days, starting at the end of the cisplatin infusion. Repeat treatment every 3 weeks.
Must receive premedication with antiemetics and appropriate hydration for cisplatin administration.
Must receive premedication with antiemetics, and appropriate hydration (prior to and after cisplatin administration). Prophylaxis for neutropenic infections should be administered.
All patients treated on the BEIZRAY-containing arms of the TAX323 and TAX324 studies received prophylactic antibiotics.
For the induction treatment of locally advanced inoperable SCCHN, the recommended dose of BEIZRAY is 75 mg/m² as a 1-hour intravenous infusion followed by cisplatin 75 mg/m² intravenous over 1 hour, on day 1, followed by fluorouracil as continuous infusion at 750 mg/m² per day for 5 days. This regimen is administered every 3 weeks for 4 cycles. Following chemotherapy, patients should receive radiotherapy.
For the induction treatment of patients with locally advanced (unresectable, low surgical cure, or organ preservation) SCCHN, the recommended dose of BEIZRAY is 75 mg/m2 as a 1 hour intravenous infusion on day 1, followed by cisplatin 100 mg/m2 administered as a 30-minute to 3 hour infusion, followed by fluorouracil 1000 mg/m2/day as a continuous infusion from day 1 to day 4. This regimen is administered every 3 weeks for 3 cycles. Following chemotherapy, patients should receive chemoradiotherapy.
All patients should be premedicated with oral corticosteroids (see PI) such as dexamethasone 16 mg per day (e.g., 8 mg twice daily) for 3 days starting 1 day prior to each BEIZRAY infusion. This premedication regimen is intended to reduce the incidence and severity of fluid retention as well as the severity of hypersensitivity reactions.
For metastatic castration-resistant prostate cancer, given the concurrent use of prednisone, the recommended premedication regimen is oral dexamethasone 8 mg at 12 hours, 3 hours, and 1 hour before the BEIZRAY infusion.

